During the LED packaging process, electrode contamination often occurs after the solid crystal baking. This paper investigates the nature of the contaminants and identifies them as solid oxide gel volatiles. Based on this, two potential causes for the adhesion of die-bonded chip electrodes are analyzed, along with corresponding solutions.
One: Electrode Contamination
In current LED packaging processes, after the solid crystal adhesive is baked and cured, contaminants can stick to the gold electrode, leading to issues such as poor wire bonding or insufficient wire pull force.
As shown in the image, a metallographic microscope was used to observe significant contaminants on the electrode surface.
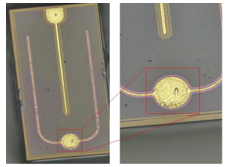
Two: Cause Analysis and Solutions
1. Contaminant Composition
Using an electronic energy spectrometer (EDS), elemental analysis was performed on the contaminated area of the chip. The elements C, O, and Si were detected, which aligns with the main components of the solid crystal glue—siloxane (H, C, O, Si). Since EDS cannot detect hydrogen, it is inferred that the contaminant contains components from the solid crystal glue.
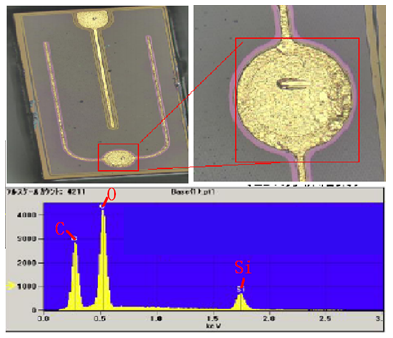
2. Cause Analysis
Based on elemental analysis, we suspect that the contaminant is the solid crystal glue, indicating some interaction between the glue and the electrode during baking.
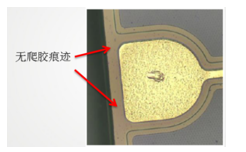
While the solid glue may spread or creep onto the electrode, no visible signs of creeping were observed around the chip, suggesting that contamination from the glue is unlikely. However, during curing, the glue may release small volatile molecules, including hydroxyl-containing cross-linkers. These molecules have high chemical activity and could be the primary cause of contamination.
How do these volatile substances adhere to the electrode?
We proposed two possible mechanisms:
1. Chemical Adsorption of Hydroxyl-Containing Cross-Linker with Chip Electrode Contaminants
Although the cross-linker does not react directly with gold, residual active contaminants from the electrode preparation process might allow the cross-linker to chemically react and cause adsorption.
2. Electrostatic Adsorption of Hydroxyl-Containing Cross-Linker with the Chip Electrode
The oxygen atom in the hydroxyl group is highly electronegative, causing the electron pair between H and O to shift toward the oxygen. This creates a dipole moment, leading to electrostatic attraction and subsequent adsorption.
2.1. Source of Chip Contaminants
Contamination may come from the chip manufacturing process or from handling during die bonding. Microscopic inspection showed no visible fingerprints, ruling out manual contamination. However, during photolithography, developers like sodium carbonate solution may leave hydroxyl groups on the electrode surface. Incomplete cleaning may lead to residual contaminants.
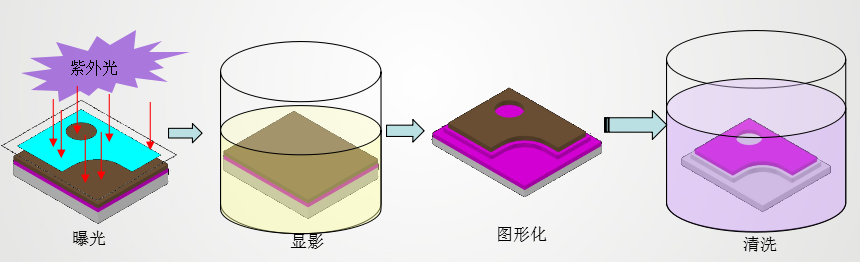
2.2. Source of Solid Crystal Glue Volatiles
The hydroxyl-containing cross-linkers in the solid crystal glue have low molecular weight and boiling point. They may volatilize during baking and form hydrogen bonds with residual hydroxyl groups on the electrode surface, eventually leading to chemical reactions.
What is a hydrogen bond?
A hydrogen bond occurs when a hydrogen atom bonded to a highly electronegative atom (like O, F, or N) interacts with another electronegative atom. This forms a special intermolecular or intramolecular interaction (XH...Y).

2.3. Reaction Process
When the hydroxyl-containing cross-linker comes into contact with residual hydroxyl groups on the electrode, hydrogen bonds form. Some of these bonds convert into covalent bonds, releasing water molecules. Over time, more cross-linkers react, leaving behind a layer of solid crystal glue containing C, O, and Si.
2.4. Experimental Verification
A clean chip was divided into two groups. One was soaked in an alkaline solution, while the other was left untreated. After applying the same amount of solid crystal glue and baking, the treated chip showed significant contamination, confirming the role of hydroxyl groups in the reaction.

2.5. Solution
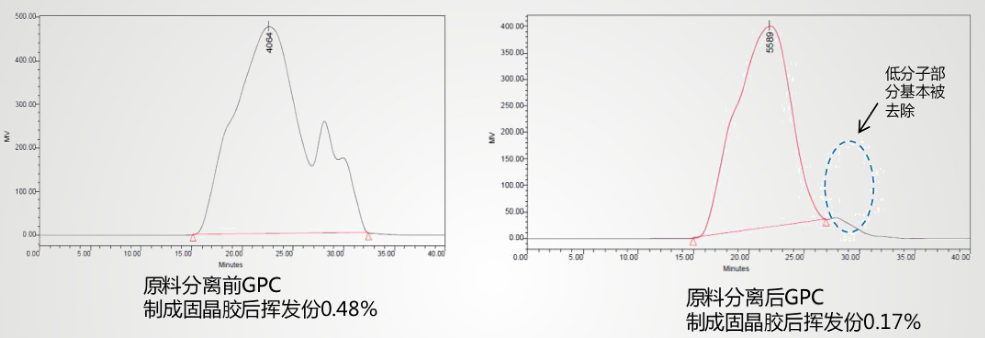
To reduce contamination, improvements are needed in both the chip and the solid crystal glue. By removing small molecules through distillation, the volatile content decreased significantly, reducing electrode pollution.

2.6. Electrostatic Adsorption Mechanism
During chip handling, static electricity can build up, especially when peeling off blue film. If operators don't use ion fans or wristbands, the chip may become charged. The hydroxyl groups in the cross-linker are polar and can be attracted to the charged electrode, leading to contamination.
2.7. Experimental Verification
Chips were tested under different conditions—some were charged, others were neutralized. After baking, the charged chips showed more contamination, proving that electrostatic adsorption plays a secondary role.
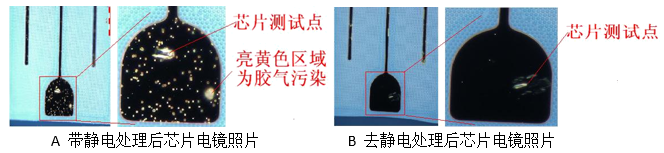
2.8. Solution
Improving operating procedures and using better-quality solid crystal glue can help reduce both chemical and electrostatic contamination.

Three: Summary
After thorough analysis, we found that the contaminant is solid crystal glue. Two mechanisms contribute to the adhesion: chemical adsorption of hydroxyl-containing cross-linkers with residual contaminants on the electrode, and electrostatic adsorption due to charge buildup. Experimental results confirmed that chemical adsorption is the main cause, while electrostatic adsorption is secondary. Solutions include improving chip preparation and solid crystal glue formulation.

References:
Liu Xing. Mechanism of silane molecule adsorption and film formation kinetics on metal surface [D]. China University of Geosciences (Wuhan), 2012.
Geng Yiping, Tang Bing, Huang Shuhuan. Advances in Adsorption of Organic Substances on Metal Surfaces[J]. Surface Technology, 2009, 38(2):70-72.
Author: Fang Huayu, Pei Xiaoming

Multi-Port Type-C Charger,Desktop Usb Charging Station,Usb Fast Charging Station,TYPE-C Fast Charging Station
shenzhen ns-idae technology co.,ltd , https://www.best-charger.com
