Related Detection Methods and Techniques for Lithium Battery Electrolytes
The previous article mainly introduced the principles and methods of detecting lithium-ion battery separators. The last one, as one of the four main components—electrolyte—is often referred to as the "blood" of lithium-ion batteries. It plays a crucial role between the positive and negative electrodes, facilitating the conduction of electrons and ensuring the high performance of high-voltage and high-power batteries. Typically, electrolytes are composed of organic solvents, lithium salts, and additives, prepared in specific ratios under controlled conditions. This article will explore the detection methods and principles of the electrolyte, guiding readers through the process of testing this critical component.

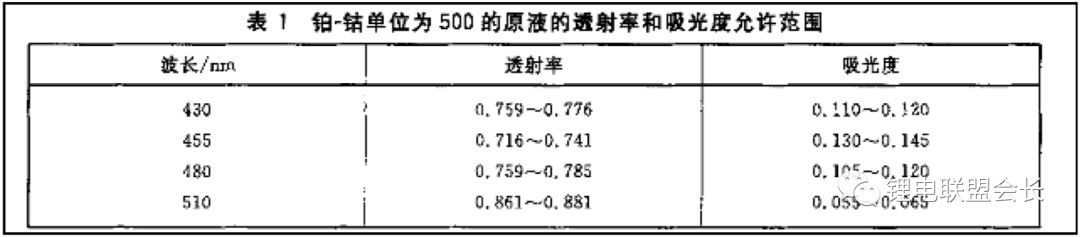
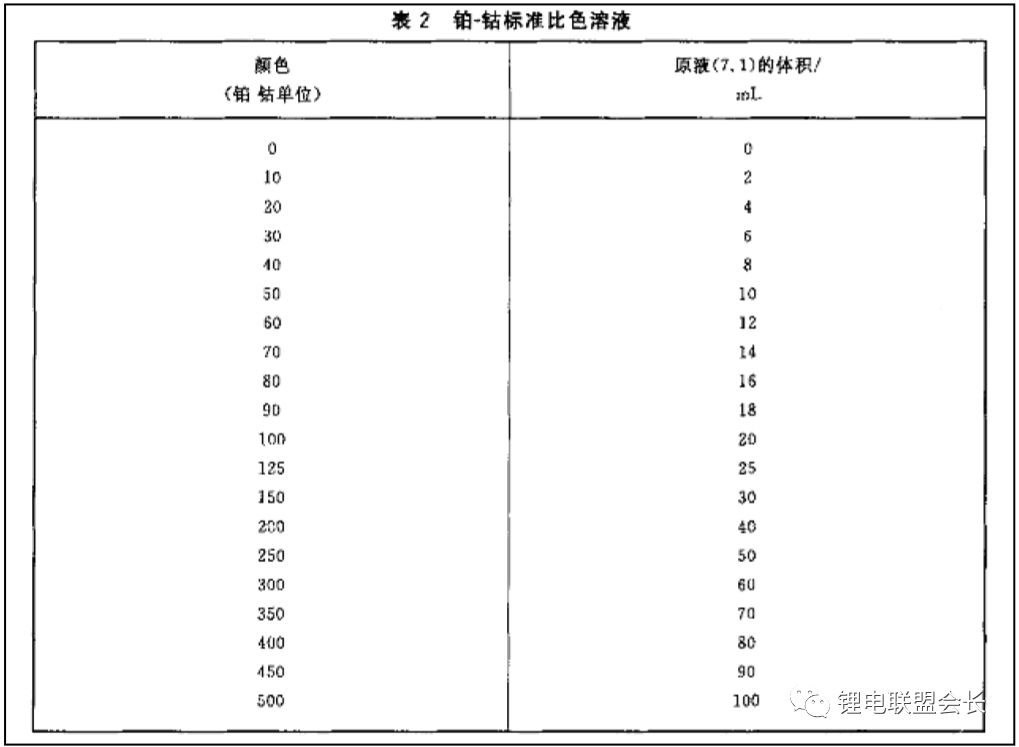
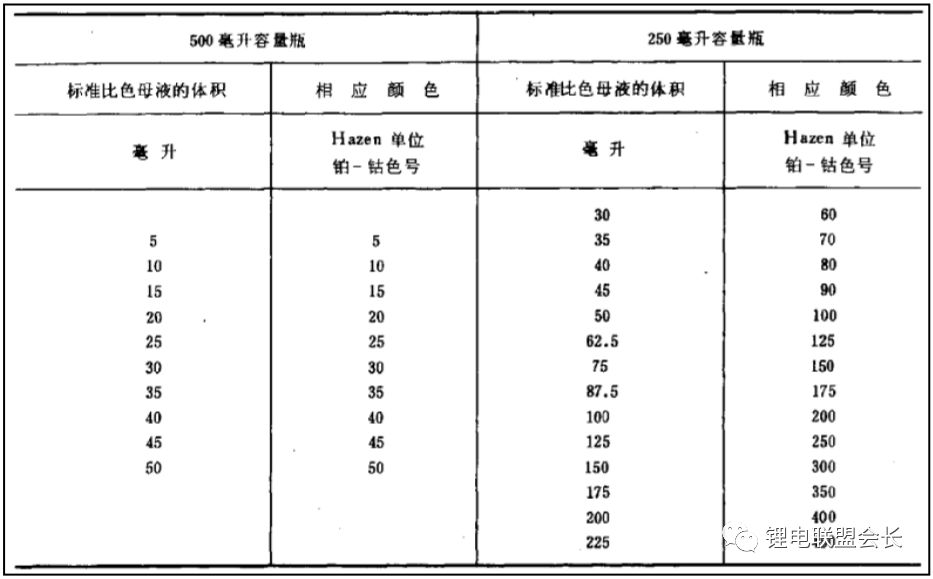
1. Appearance: The appearance test involves observing the color of the electrolyte. Most electrolytes are clear and colorless, but there are specific standards for this test. For example, GB/T 9282.1-2008 specifies the use of a platinum-cobalt scale to measure the color of transparent liquids. This is done by preparing standard solutions and measuring their absorbance and transmittance using a spectrophotometer at different wavelengths. A series of standard colorimetric solutions are then prepared and compared with the sample to determine its color grade.
2. Moisture Content: The Karl Fischer method is commonly used for moisture testing, which was previously discussed and won't be repeated here. Interested readers can refer back to the earlier post for more details.
3. Free Acid: Due to the presence of LiPF₆ in the electrolyte, hydrofluoric acid (HF) may be released when it comes into contact with water. During production, transportation, and usage, the electrolyte is inevitably exposed to air or moisture. Therefore, free acid testing is typically conducted before the electrolyte leaves the factory or is injected into the battery. The method is simple, involving acid-base titration. Some recent patents have explored the selection of specific bases, though the basic principle remains the same.
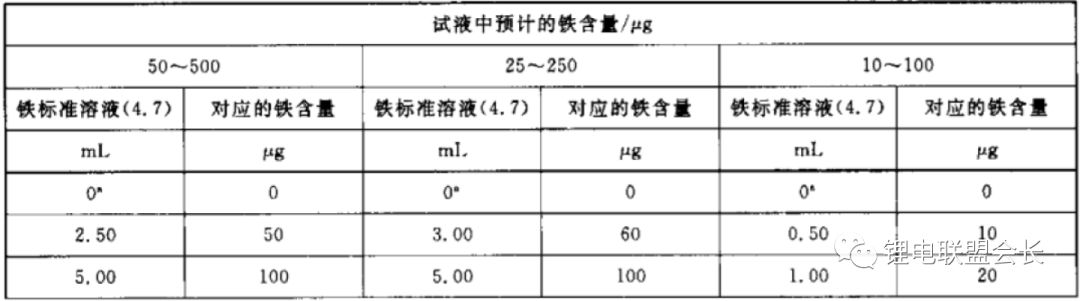
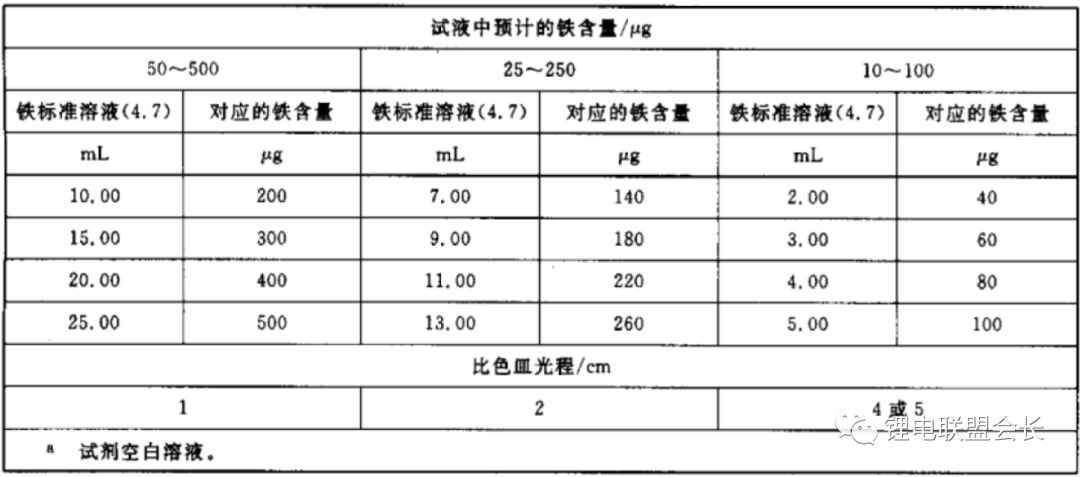
4. Iron Content: During the preparation and transportation of the electrolyte, iron impurities can be introduced, leading to performance degradation in lithium-ion batteries. The standard method for determining iron content is the porphyrin spectrophotometric method, based on reducing ferric ions to ferrous ions with ascorbic acid. At pH 2–9, ferrous ions react with phenanthroline to form an orange-red complex. The absorbance at 510 nm is measured using a spectrophotometer, and the iron content is determined from a standard curve.
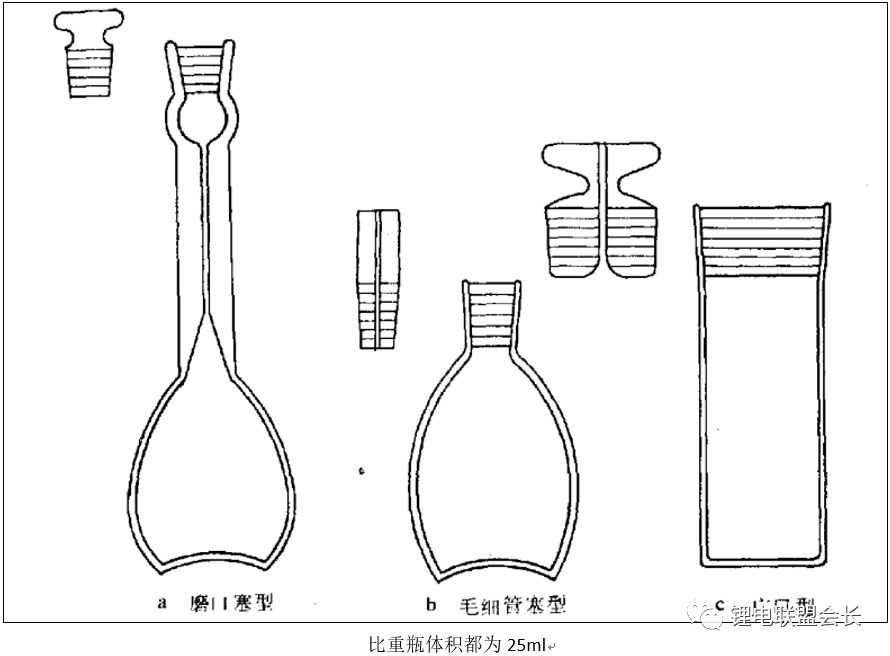
5. Density: The density of the electrolyte is generally measured using the pycnometer method according to GB/T 2540. The appropriate pycnometer is selected based on the sample, and the liquid is placed inside and heated in a constant temperature bath until no changes occur. Excess liquid is removed with filter paper, and the surface is wiped clean. The weight is then measured, and the density is calculated using the formula provided.
6. Conductivity: Conductivity measures how easily charge flows through a material and is a key parameter that affects the power performance of the battery. It is typically measured using a conductivity meter.
7. Color: The color of the electrolyte is described by brightness and chromaticity. Chromaticity refers to the color excluding brightness, reflecting the hue and saturation. It is measured using the platinum-cobalt color number method, as specified in GB/T 3143. The process is similar to the appearance test, with results obtained via spectrophotometry and comparison with standard samples.
8. Chloride Ion Content: This is determined using a silver nitrate titration method, which is straightforward and does not require detailed explanation here.
9. Sulfate Ion Content: In a hydrochloric acid medium, strontium ions react with sulfate ions to form insoluble barium sulfate. When the sulfate concentration is low, the solution becomes cloudy, and the turbidity is compared visually with a standard to determine the result.
10. Impurity Content: To detect elements such as K, Na, Fe, Ca, Pb, Cu, Zn, Ni, and Cr, inductively coupled plasma optical emission spectrometry (ICP-OES) is used. The sample is introduced into the atomization system via argon gas, where it is atomized and excited in the plasma. The emitted electromagnetic radiation is quantified based on the relationship between radiation lines and element concentrations. This method was briefly mentioned in a previous article.
11. Electrochemical Properties: These are evaluated by assembling the battery and conducting a series of electrical performance tests. This is a familiar process for most professionals in the lithium-ion battery field, so it is not elaborated further here.
Summary:
As lithium-ion battery technology advances, the electrolyte is expected to evolve from a liquid state to semi-solid and solid forms, while also moving toward higher voltages. Research is ongoing on flame-retardant, low-calorific, and high-safety electrolytes. With continued industry development, more advanced electrolytes will likely become widely used in lithium-ion batteries. This concludes the series on detection methods for the four major materials. I hope you continue to follow along and stay updated on future developments. Thank you!
Transformer Accessories,Innovative Chip Radiator,High Efficiency Chip Radiator,High Temperature Resistant Transformer Bushing
Tianhong Electric Power Technology Co., Ltd , https://www.tianhongtransformer.com
